IgA 血管炎について
Abstract
Immunoglobin A (IgA) vasculitis (IgAV), formerly called the Henoch-Schönlein purpura (HSP), is a small vessel vasculitis, characterized by IgA1-dominant immune deposition at diseased vessel walls. IgAV is the most common form of vasculitis in children; typical symptoms include palpable purpura, arthritis or arthralgia, abdominal pain, and hematuria or proteinuria. Galactose-deficient IgA1 is detected in the tissues of the kidney and skin in patients with IgAV; it forms immune complexes leading to subsequent immune reactions and injuries. This report provides the recent advances in the understanding of environmental factors, genetics, abnormal innate and acquired immunity, and the role of galactose-deficient IgA1 immunocomplexes in the pathogenesis of IgAV.
Keywords: IgA vasculitis, IgA, pathogenesis, kidney, skin
1 Introduction
Immunoglobin A (IgA) vasculitis (IgAV), formerly called the Henoch-Schönlein purpura (HSP), is a small vessel vasculitis, characterized by IgA1-dominant immune deposition at diseased vessel walls (1). It may occur as systemic or single-organ limited vasculitis. The skin, kidney, gastrointestinal tract, and joints are often involved (1).
IgAV is the most common form of vasculitis in children, with an annual incidence rate of ~20 per 100,000 children (2–7). Typical symptoms include palpable purpura, arthritis or arthralgia, abdominal pain, and hematuria or proteinuria. In most cases, the disease is self-limited, but relapse is common. Gastrointestinal involvement occurs in 10%–40% of patients, and renal involvement occurs in 10%–55% of patients. Altogether, they are the principal causes of morbidity and mortality (8–10). IgAV is relatively rare with an incidence of 0.8–2.2 per 100,000 person-years in adults (2, 11). Aging negatively impacts the severity and outcome of the disease in adult patients with IgAV (12). Younger patients are more frequently involved with the joint and gastrointestinal tract, whereas old patients are at increased risk of severe purpura and glomerulonephritis, including end-stage kidney disease (ESKD) (12–16).
Although the epidemiology, clinical manifestations, and outcomes of IgAV are well established, our understanding of the pathogenesis of IgAV is still limited. In the past decade, efforts have been made to further understand IgAV. Identification of environmental and genetic factors and recognition of aberrant IgA may shed light on the pathogenesis of IgAV. Herein, we present recent advances in the understanding of the environmental factors, genetic factors, abnormal innate and acquired immunity, and the role of galactose-deficient IgA1 immunocomplexes in the pathogenesis of IgAV.
2 Environmental Factors and IgAV
The seasonal tendency of IgAV has been reported in several extensive cohort studies (17–19). The onset is more frequent during September to April and less common during summer. Recently, according to a Croatian study, geospatial clustering of IgAV along the course of Drava and Danube rivers, similar to the spatial distribution of Balkan endemic nephropathy triggered by daily exposure to environmental factors was observed (aristolochic acid) (20, 21). The temporal and geospatial associations imply that environmental factors are involved in the pathogenesis of IgAV.
The history of infection of the upper respiratory tract or the history of exposure to antigens from certain foods, insects, drugs, or vaccines can usually be found before the onset of IgAV, suggesting that infection or exposure to mucosal antigen may trigger the pathogenesis of IgAV (1, 22). This may also explain the regional and seasonal distribution of IgAV (23). The most commonly reported pathogens related to IgAV are group A Streptococcus, parainfluenza virus, and Human Parvovirus B19 (8, 17, 18). Helicobacter pylori is also associated with the disease. Patients with H. pylori have increased the risks of IgAV; H. pylori eradication therapy contributed to the rapid improvement of IgAV (24, 25). Since the COVID-19 outbreak, several cases of COVID-19 related IgAV have also been reported (26–28). The serum anti-COVID-19 IgA but not IgG was detected in patients with IgAV, and endothelial injuries may be involved (27). Besides pathogens, various vaccines, including the live attenuated vaccines of measles, mumps, rubella, and the inactive antigen vaccines of influenza or hepatitis B, may trigger IgAV (29).
The mechanism of pathogen or mucosal antigen-related IgAV is unclear; however, theoretically, it can be pointed to the modulation of mucosal immunity, including galactose-deficient IgA1 (Gd-IgA1) production (30–33). It was postulated that pathogen and mucosal antigens may trigger immune responses through molecular mimicry, increased intestinal permeability, and abnormal production of IgA1 results in subsequentially immune dysfunction (see details in the following section) (34, 35).
3 Genetics and IgAV
According to epidemiological studies across the world, incidence rates differ among races. In a UK study, Asians showed the highest incidence at 24.0(18.2–31.2) per 100,000 per year, whereas black people possessed the lowest incidence at 6.2 per 100,000 per year. Likewise, in a smaller population American study, the Hispanic children possessed a higher incidence (8.6 per 10,000 children) than African American and Caucasian children (0.9 per 10,000) (2, 3). In both studies, black people had a lower incidence than other ethnicities. However, epidemiological investigations were conducted via different methods, and further multicountry studies with uniform methodologies are needed to confirm whether ethnic variation is the risk factor of IgAV.
The genetic factors are related to IgAV (36). Human leukocyte antigen (HLA)-B35 and HLA-DRB1*01 alleles are associated with susceptibility to IgAV (37, 38). Especially, HLA-DR1*0103 is strongly associated with increased susceptibility to IgAV (39). Polymorphism in genes encoding cytokines was found to be associated with manifestations of IgAV (36). Polymorphism of IL-8, a cytokine that plays a central role in the recruitment of neutrophils, associates with an increased risk of cutaneous IgAV (40). IL-1 polymorphism was found to be associated with the severity and outcome of IgAV-N (41, 42). Polymorphism in enzyme encoding genes may also affect the synthesis of Gd-IgA1 (43, 44). Mutation of MTHFR gene (encodes methylenetetrahydrofolate reductase), and factor V Leiden may be associated with clinical symptoms (36, 45) (46). Association of Mediterranean fever (MEFV) gene mutation and IgAV has been reported in Mediterranean population (47–53). IgA vasculitis can occur at 2.7-7% patients with familial Mediterranean fever (46). These patients tends to have less IgA deposits than those with IgAV alone (54). MEFV gene encodes pyrin, a modulator of innate immunity, and pathogenic MEFV mutation leads to altered innate immune system inflammation and thus increases the susceptibility of vasculitis (55). The genetic susceptibility of IgAV may vary among different ethnic populations, and further investigation conducted across the world is warranted to confirm the role of these genes in different populations.
4 Immunopathogenesis of IgAV
As the name of the disease indicates, the most notable pathological feature of IgAV is IgA1-dominant IgA deposits in the vessel walls. Aberrant IgA and IgA complexes are considered to play a central role in the immunopathogenesis of IgAV.
IgAV shares many similarities with another IgA mediated disease, IgA nephropathy. (IgAN). IgAN is defined by the IgA-dominant deposits in mesangial area of the kidney. IgAV and IgAN shares many similarities in clinical and pathologic features. Especially, it can hardly be distinguished from renal-limited IgAV (56, 57). Similarities and differences between IgAV with nephritis and IgAN are presented in Table 1 (58, 59).
Table 1.
Differences and similarities between IgAV with nephritis and IgAN(48, 49).
| Characteristics | IgA Vasculitis with nephritis | IgA Nephropathy |
|---|---|---|
| Onset | Children younger than 10 years of age | More common in adulthood |
| Organs involved | Systemic or single-organ limited (skin, kidney, joint, gastrointestinal tract, etc. ) | Kidney |
| Disease course | Acute, with spontaneous resolution | Chronic and progressive |
| Gender preference | More common in male (about 2:1) | |
| Abnormal IgA | Galactose-deficient IgA1 | |
| Light microscopy | Mesangial proliferation, endocapillary hypercellularity, segmental sclerosis, crescents | |
| Immunofluorescence microscopy | IgA1 dominant deposits in the glomerular mesangium | |
| Outcome | More severe in adults | |
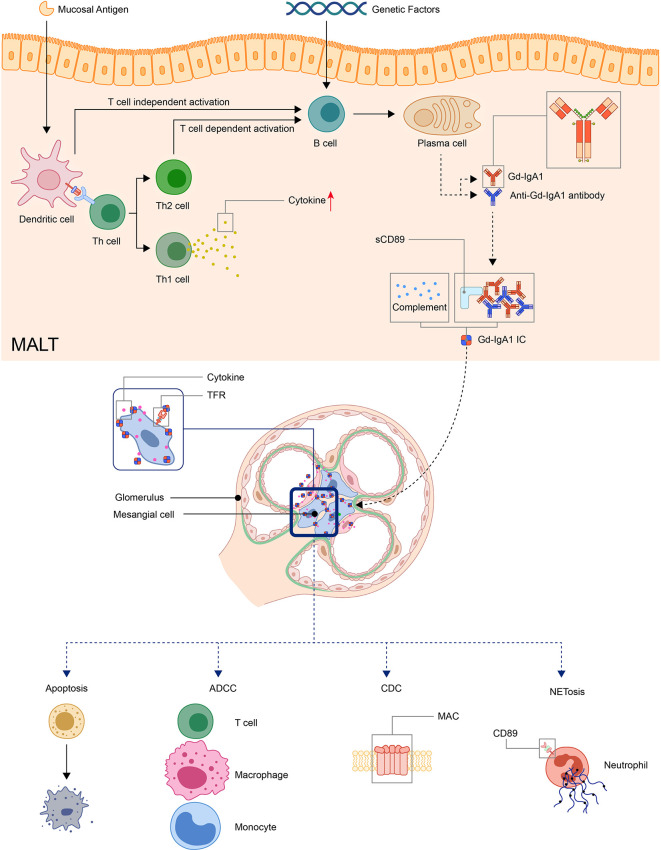
Detection of clustered onset in twins, one with IgAN and the other with IgAV-N, further confirmed the relationship between two diseases (60). So it has long been speculated that IgAV and IgAN may have similar pathogenic mechanisms (17). A widely accepted hypothesis for the pathogenesis for IgAN is a multi-hit model proposed by Novak J. et al (61). The model was originally used in IgA nephropathy but later found applicable in IgAV (62, 63). In this model, the first and second hit is the production of Gd-IgA1 and autoantibodies against Gd-IgA1, the third hit is the formation of Gd-IgA1 containing immune complexes, and finally, the fourth hit is the deposition of immune complexes in tissue activates the inflammatory process that results in organ injury (62–65) ( Figure 1 ).
Figure 1.
Model: pathogenesis of IgA vasculitis. The mucosal antigen can activate B-cells in MALT through T-cell-dependent or independent ways. The latter activates B-cells through TLR pathways. With genetic factors, the activated B-cells become plasma cells and produce Gd-IgA1. Gd-IgA1 and anti-Gd-IgA1 autoantibodies form circulating immune complexes together with other components (including sCD89 or complements). Then, the immunocomplex deposit at organs and activate inflammatory responses. In the kidney, the immunocomplex can activate mesangial cells through TfR, leading to the apoptosis of renal cells and recruitment of inflammatory cells. (ADCC, antibody-dependent cytotoxicity; CDC, complement-dependent cytotoxicity; Gd-IgA1, Galactose-deficient IgA1; MAC, membrane attack complex; MALT, Mucosa-associated lymphoid tissue; NET, neutrophil extracellular traps; TfR, transferrin receptor).
4.1 Galactose-Deficient IgA1 and Its Autoantibodies
The hallmark histologic feature of IgAV is leukocytoclastic vasculitis with IgA immune complex deposits in small vessels. Alterations in the O-linked glycosylation of IgA1 are found in patients with IgAV, and higher serum Gd-IgA1 level is related to a higher risk of kidney involvement, although not with disease severity (63, 66, 67). Gd-IgA1-dominant IgA deposits were detected in the kidney, skin, and gastrointestinal tract biopsies, and it was considered an important factor in the immunopathogenesis of IgAV (1, 68).
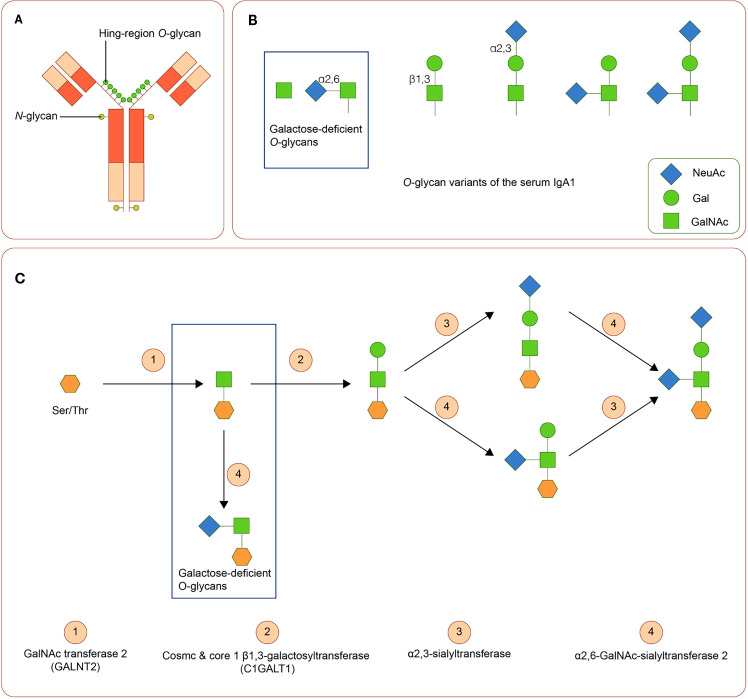
IgA is a Y-shaped immunoglobulin with two heavy chains and two light chains. A short segment of amino acids forms the hinge region in the central part of the heavy chains. In general, according to its location, IgA can be subdivided into the serum or the secretory IgA. Serum IgA1 is predominantly produced by B-cells in the bone marrow, whereas secretory IgA is primarily generated by activated B-cells near the mucosae and the exocrine glands (69). Two subclasses of IgA, namely, IgA1 and IgA2, are produced in a ratio of 5:1. The hinge region of IgA1 usually contains three to six O-linked glycan sites ( Figures 2A, B ). At these sites, galactose (Gal) and N-acetylgalactosamine (GalNAc) with or without sialic acid (N-acetylneuraminic acid, Neu5ac) can attach to oxygen atoms of serine or threonine residues through glycosidic linkages (70, 71)
Figure 2.
IgA1 Oglycans (A) Structure of human IgA1. The hinge region of IgA1 usually contains three to six O-linked glycan sites. (B) Variants of IgA1 O-glycan. (C) Synthesis of human IgA1 O-glycans. Upregulated α-N-acetylgalactosaminide α2,6-sialyltransferase 2 and down regulated core 1 β1,3-galactosyltransferase (and Cosmc) can lead to the increase of galactose-deficient IgA1.
Variation in activities or expression of critical enzymes that catalyze O-glycosylation of IgA1 in B-cells may lead to Gal deficiency of IgA1. Three enzymes are critical in O-glycosylation of IgA1: polypeptide N-acetylgalactosaminyltransferase 2 (GALNT2) attaches GalNAc to serine or threonine, core 1 β1,3-galactosyltransferase (C1GALT1), with its chaperone Cosmc links Gal to GalNAc, and sialyltransferases complete the glycan structure by attaching sialic acid to Gal or GalNAc residues (α2,3-sialyltransferase for Gal and α2,6-GalNAc-sialyltransferase 2 for GalAc) (72). Downregulated expression of C1GALT1 and upregulated α2,6-GalNAc-sialyltransferase 2 are associated with the production of Gd-IgA1 (73). ( Figure 2C ). Cytokines, including IL-6 and IL-8, participate in the regulation of these enzymes (74). Polymorphisms in the genes of these enzymes are also involved in the synthesis of Gd-IgA1 (43, 44, 75–77).
As stated above, the onset of both IgAV and IgAN typically follows episodes of respiratory infection, which indicates that mucosal antigens may play a role in the process. The mucosal immune response can induce Gd-IgA1 production by peripheral B-cells. In mucosa-associated lymphoid tissue (MALT), activated B-cells produce IgA in T-cell-dependent or -independent manners. The latter one involves the interaction between B-cell and dendritic cell and the Toll-like receptor (TLR) pathway. Tonsillar B-cell activation through TLR on dendric cells may lead to the production of Gd-IgA1 in patients with IgAN, and tonsillectomy can reduce the serum levels of Gd-IgA1 (78, 79). TLR9 and the A proliferation-inducing ligand (APRL), IL-6 mediated pathways, and the TLR7-GALNT2 axis are involved in the synthesis of Gd-IgA1 (79, 80). TLR2 and TLR4 are upregulated in patients with IgAV and IgAN, and the level of TLR4 expression is related to proteinuria (81, 82).
The presentation of autoantibodies can be induced by the residues in Gd-IgA1 or mucosal antigen that mimic the structure of Gd-IgA (83). In Gd-IgA, the abnormal glycosylation exposes nearby GalNAc residues, and the latter can become neoepitopes (84). An elevated level of IgG autoantibodies specifically against Gd-IgA1 was detected in IgAV-N patients and is associated with disease activity, whereas in those without nephritis, it is similar to the control groups (85). There are other isotypes of autoantibodies against Gd-IgA1, but their role is poorly understood.
Reduced galactosylation in the O-glycan site of the hinge region of IgA1 was detected in patients with IgAV, especially IgAV-N (63, 86, 87). Recent findings of Gd-IgA1in the cutaneous lesions and clinically uninvolved skin in skin-limited IgAV further confirmed its role in IgAV (62). The serum level of Gd-IgA1–specific IgG is associated with disease activity and renal involvement (85). Targeted release formulation of budesonide that can target Payer’s patches in the ileum and suppresses the gastrointestinal immune system can decrease the level of Gd-IgA1 and may be a potential treatment for IgAV (88–90).
4.2 Formation of Immune Complex
The formation of the Gd-IgA1 immune complex is a critical step in the pathogenesis of IgAV. The proliferation of mesangial cells can be stimulated by Gd-IgA1 immune complexes, but not isolated Gd-IgA1 (91). The levels of serum Gd-IgA1 are heritable, and healthy relatives of patients may have elevated serum Gd-IgA1 levels without clinical symptoms, suggesting that Gd-IgA1alone is insufficient to cause IgAV; other factors, such as the formation of Gd-IgA1 immune complexes, are also critical for the pathogenesis of IgAV (92, 93).
Gd-IgA1 can self-aggregate or bind to its autoantibodies, thereby forming circulating immune complexes (CIC). IgA1 containing CIC is detected in patients with IgAV, although heterogeneity in CIC composition was observed (87). Patients without nephritis tend to have IgA1-CIC with smaller molecular mass, whereas that of those with IgAV-N is larger (usually IgA1-IgG-CIC) (85, 94, 95).
Fc alpha receptor I (FcαRI, also known as CD89) is a transmembrane IgA receptor in myeloid cells and can be released as a soluble form (sCD89) after cleavage of the extracellular domain. CD89 is involved in the deposition of IgA-CICs in the kidney. The serum levels of the sCD89-IgA1 complex are higher in IgAV patients than those in health control (96, 97). Transgenic mouse model expressing human CD89 and IgA1 suggested that IgA1 and sCD89 forms CICs that deposit at mesangial cells. sCD89 has a low affinity for monomeric or dimer IgA but a high affinity for IgA immune complexes (98). It can bind to IgA1 and further increase the size of IgA1-CIC. The interaction between CD89 and IgA-containing immune complexes results in phagocytosis, antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, production of reactive oxygen species, and cytokines that lead to the destruction of the tissue (98–101).
4.3 Deposition of Immune Complex
The formation of IgA-CIC hinders the liver clearance of these immune complexes, and overloaded IgA-CIC can deposit at vessel walls (102–105). Deposition of Gd-IgA1 containing immunocomplex can be found in small vessel walls in the skin as well as the kidney and mesangial cells (62). The serum level of Gd-IgA1 is not correlated with the intensity of Gd-IgA1 deposits in the kidney and skin, suggesting that factors other than size and amount of CIC influence the deposition of immune complexes and other mechanisms are involved in the deposition of Gd-IgA1 (65).
4.3.1 Deposition of Immune Complex in the Kidney
Collaboration between IgA-sCD89, transferrin receptors(TfR), and transglutaminase 2 is required in renal injury (106). TfR, also known as CD71, are a group of IgA1 receptors expressed in mesangial cells. In IgAV-N, TfR expression is increased. IgA1 isolated from patients with IgAV induces increased expression of TfR, activates PI3K/Akt/mTOR pathway, and stimulates the proliferation of human mesangial cells (107). Hypogalactocylation in IgA1 and large molecular sizes enhance the affinity of immune complexes to TfR in mesangial cells and promotes the deposition in mesangial cells and subsequent activation of the IgA receptor (102). β-1,4-galactosyltransferase, as an IgA receptor in mesangial cells, also participates in the deposition of IgA (108).
In patients with IgAV, immune complexes activate mesangial cells and induce mesangial proliferation, expression of proinflammatory cytokines and chemokines (IL-6, IL-8, TNFs, and MCP-1), and apoptosis of podocytes and tubular epithelial cells. These results in the recruitment of inflammatory cells, and further augmenting the injury in the kidney (61, 71, 109, 110).
4.3.2 Deposition of Immune Complex in the Skin
It is controversial whether Gd-IgA1 participates in the pathogenesis of IgAV patients without nephritis. Traditionally, it is believed that IgG-containing CICs were found only in IgAV patients with nephritis, which tends to have poorer outcomes. And some clinical studies suggested that the serum levels of Gd-IgA1 or Gd-IgA1-CIC in IgAV patients without nephritis are the same against healthy controls (63, 86). However, in a recent study, Gd-IgA1 was detected using KM55 staining in the skin of IgAV patients without nephritis (62). The result suggested that Gd-IgA1 are important in both systemic and organ limited IgAV. Nevertheless, KM55 staining is a lectin-independent approach used to detect Gd-IgA1 (111). Gd-IgA1 deposits were also found using the staining method in renal biopsies in other secondary IgA nephropathy and incidental IgA deposits without nephritic syndrome (112, 113). The conclusion needs to be carefully explained and to be confirmed using different staining methods.
4.4 The Role of Complements
The activation of complements is also involved in tissue injuries in patients with IgAV. Elevated C3a, C5a, and Bb fragments, and C3 and C5-9 deposits, indicate the activation of the alternative pathway (114). C4d and C5b-9 deposits in the kidney are associated with poor renal outcomes (115).
Activation of complements through the mannose-binding lectin pathway was reported in IgA-V (116–118). A recent case report suggests that a monoclonal antibody against mannose-binding lectin serine peptidase 2, an inhibitor of the lectin pathway, can be used to treat IgAV-N (119). The activated complements induce upregulated expression of cytokines and recruit inflammatory cells (83, 93).
4.5 Inflammatory Cells in IgAV
Together with IgA immune complexes, infiltration of inflammatory cells can be observed around vessel walls, suggesting that these cells may be involved in tissue injury of IgAV.
4.5.1 Neutrophils and NETs
Neutrophils are predominant cells in inflammatory infiltration in cutaneous and gastrointestinal biopsies from patients with IgAV. In neutrophils, the cross-link induces the release of neutrophil extracellular traps (NETs) and neutrophil chemoattractant leukotriene B4 that may augment the damage in a positive feedback manner (120, 121). NETs are web-like chromatin structures that play an important role in the clearance of pathogen. NETs are released through NETosis, and the latter is triggered by immune receptors through mediators, including reactive oxygen species (122). The immune complex can induce NETosis via FcγRIIIB or CD89 (123, 124). NETs and reactive oxygen species are increased in both superficial and deep dermal perivascular tissue in IgAV (125).
4.5.2 T-Cells
T-cells are involved in tissue damage in IgAV (126, 127). There are two major populations of T-cells, CD4 and CD8 T-cells. CD4 T-helper (Th), CD4 regulatory T-cells (Treg), and CD8 cytotoxic T-lymphocytes (CTL) are important subsets of T-cells. Activation of circulating CTLs is found in patients with IgAV, and increased CTLs in glomeruli contribute to kidney injuries in IgAV (126). CXCR3 is highly expressed in effector T-cells. CXCR3-expressing T-cells are found recruited in the skin and kidneys of patients with IgAV, and the degree of infiltration of T-cell in the kidney is associated with the severity of kidney impairment (127).
Th17 is a subset of CD4-positive T-cells that produces IL-17. In patients with active IgAV, serum levels of IL-17 were elevated, and the number of Th17 in peripheral blood increased (128, 129). Interestingly, a monoclonal antibody against IL-17A (secukinumab) can trigger IgAV (130). It is speculated that the secukinumab breaks the balance of regulators of Th17 cells, leading to increased proinflammatory cytokines that induce IgAV. A recently reported case of IgA vasculitis complicated by psoriasis vulgaris may help explore the mechanism. In this patient, skin lesions of IgAV appears in sparing area of psoriasis (131). The patient adopted maxacalcitol, which can induce regulatory T-cells, and resident regulatory T-cells in psoriatic lesions may suppress the activity of IgAV.
Treg is a subset of T-cells that can suppress or regulate immune responses. Type-1 T regulatory (Tr1) cells can produce high levels of IL-10 and TGF-β, and are thought to regulate local immune microenvironments wherein specific antigens exist (132, 133). In patients with IgAV, the suppressive function of Tr1 is impaired, and the number of Tr1 in peripheral blood during remission is negatively associated with the relapse of the disease (134).
4.5.3 Others
TNF-α is a cytokine mainly secreted by monocyte/macrophages. Serum TNF-α is elevated in patients with IgAV, and the level is associated with disease severity, suggesting the participation of other inflammatory cells (135). Paradoxically, TNF-α inhibitors may induce IgAV in patients with inflammatory bowel disease or psoriasis, but the causality is not confirmed (136–138). A possible explanation is that TNF-α inhibitors form immune complexes with endogenic TNF-α and deposits at vessel walls.
Conclusion and Perspectives
Genetic factors, disrupted mucosal immunity, and immune complexes with abnormal IgA or IgA antibodies are essential in the pathogenesis of IgAV. Nevertheless, the pathogenic mechanisms of IgAV are far from being completely understood, and further investigations are required. For example, mucosal immunity, especially gastrointestinal lymphoid organs, may play a key role in the pathogenesis of IgAV. However, the cellular and molecular mechanisms are unclear; how pathogen or antigen triggers immune responses and what roles those cytokines recognized as biomarkers play in the pathogenesis is not known. Furthermore, the role of IgA in the development of vasculitis needs to be further explored. An in-depth understanding of how acquired and innate immunity participates in the pathogenesis of IgAV may provide the possibility of targeted treatments. Deciphering the molecular pathogenesis of IgAV can provide a platform to identify new targets for the treatment of the disease.
Author Contributions
JHC, JW, and JC were responsible for the conception and design of the review. YS and XH drafted the manuscript. GY and JQ revised the manuscript. All authors contributed to the article and approved the submitted version.