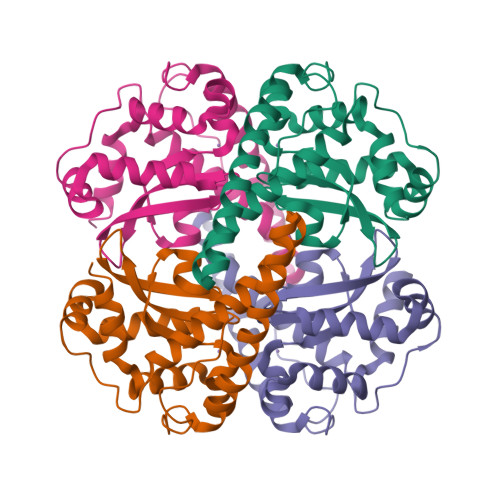
Contribution of human manganese superoxide dismutase tyrosine 34 to structure and catalysis.
Perry, J.J., Hearn, A.S., Cabelli, D.E., Nick, H.S., Tainer, J.A., Silverman, D.N.
(2009) Biochemistry 48: 3417-3424
- PubMed Abstract:
Superoxide dismutase (SOD) enzymes are critical in controlling levels of reactive oxygen species (ROS) that are linked to aging, cancer, and neurodegenerative disease. Superoxide (O(2)(*-)) produced during respiration is removed by the product of the SOD2 gene, the homotetrameric manganese superoxide dismutase (MnSOD).
Here, we examine the structural and catalytic roles of the highly conserved active-site residue Tyr34, based upon structure-function studies of MnSOD enzymes with mutations at this site. Substitution of Tyr34 with five different amino acids retained the active-site protein structure and assembly but caused a substantial decrease in the catalytic rate constant for the reduction of superoxide. The rate constant for formation of the product inhibition complex also decreases but to a much lesser extent, resulting in a net increase in the level of product inhibited form of the mutant enzymes. Comparisons of crystal structures and catalytic rates also suggest that one mutation, Y34V, interrupts the hydrogen-bonded network, which is associated with a rapid dissociation of the product-inhibited complex. Notably, with three of the Tyr34 mutants, we also observe an intermediate in catalysis, which has not been reported previously. Thus, these mutants establish a means of trapping a catalytic intermediate that promises to help elucidate the mechanism of catalysis.
好中球や単球などの貪食細胞はNADPH oxidase/NOXを用いてO2--を産生し
一般に細胞虚血が起きると細胞内のATPが分解されて、ヒポキサンチンC5H4N4Oを基質としてXO/xanthine oxidaseが働いてO2--ga産生される
ここでSODは2O2--に作用してH2O2を産生する
フリーラジカルやROSが過剰になり酸化ストレスといわれる状態になると、
脂質、糖、タンパク質、核酸が攻撃されて酸化され、蛋白の変性、酵素の不活化、DNA異常が亢進する。






